Features
Copper on the surface
Date: 2019-08-15 10:46:06.0
Author: Jon Evans
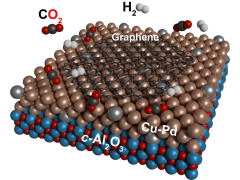
This illustration shows how graphene grows
on the novel copper-palladium surface.
Image: E. Moreno-Pineda, KIT.
Copper-based catalysts can electrochemically convert carbon dioxide into a range of useful chemicals and materials, just not particularly efficiently. Now, two new studies suggest a simple way to make these catalysts more efficient, by adding other metals.
The main problem with converting carbon dioxide into useful chemicals and materials is that carbon dioxide isn’t very reactive, which means it first needs to be reduced to carbon monoxide, which is more reactive. But the copper-based catalysts that are good at reducing carbon dioxide aren’t as good at reacting the resultant carbon monoxide with substances such as water or hydrogen to produce useful chemicals and materials, and vice versa.
This means that converting carbon dioxide into useful chemicals and materials is currently a two-step process that involves two different catalysts, often two different copper-based catalysts. But it would obviously be easier and simpler to do the whole thing in a single step with just one catalyst, and this is what two research groups have now accomplished by combining copper with another metal. One group has developed a catalyst that can convert carbon dioxide into methane, while the other has developed a catalyst that can convert it into graphene.
The methane catalyst was developed by a team of scientists from the US, China and Taiwan, and comprises a nanostructured silver surface doped with copper. For while copper can electrochemically reduce carbon dioxide to carbon monoxide, so, under certain conditions, can metals such as gold and silver. This means that a catalyst that combined gold or silver with copper could theoretically use the gold or silver to reduce carbon dioxide to carbon monoxide and then use the copper to convert the carbon monoxide into useful chemicals such as methane.
Using computational modelling, the scientists set out to design a catalyst that would do this, finding that a nanostructured silver surface doped with a specific amount of copper should prove particularly effective, which they then confirmed experimentally. As they report in a paper in Nature Communications, when used with an aqueous electrolyte their catalyst could convert carbon dioxide directly into methane with an efficiency of 60%, which is much higher than can be achieved by a copper surface on its own.
"In this work the primary novelty is to combine these two in a configuration so that several steps of reaction could occur in one system," said co-lead researcher Bingjun Xu from the University of Delaware. "We systematically modified the composition, the silver-to-copper ratio in the structure. Those are key to the selectivity and ability to combine the reactions."
Copper is a versatile substance and another of its abilities is being able to catalyze the growth of the advanced material graphene, a single layer of carbon atoms in a honeycomb pattern that boasts impressive strength and conductivity. This happens when a carbon-containing compound such as methane is passed over a copper surface at temperatures of around 1000°C. Doing the same thing with carbon dioxide has proved more difficult, but now a team of scientists from Germany has succeeded, using a surface made from copper and palladium.
As the scientists report in a paper in ChemSusChem, they tried various different arrangements of copper and palladium, including islands of palladium on a surface of copper and a region of copper next to a region of palladium. The surface that worked best turned out to comprise a layer of copper with a few palladium atoms dispersed through it, such that copper atoms comprised at least 82% of the surface.
When the scientists passed a mixture of carbon dioxide and hydrogen over this surface, they found that a layer of graphene would spontaneously grow on it. They think that the carbon dioxide reacts with the hydrogen on the surface to produce water and various carbon-containing compounds, including carbon monoxide and methane, which are then converted into graphene.
Both research groups suggest that similar approaches to combining copper with other metals could produce more catalysts able to convert carbon dioxide into methane, graphene and other useful chemicals and materials in a single step.
The views represented here are solely those of the author and do not necessarily represent those of John Wiley and Sons, Ltd. or of the SCI.
Displaying 2 keywords used to tag this article:
- Jules Audemars-Australia Best Quali
- DG6582 Mens Moncler Down Jackets Gr
