Features
Solid and heavy
Date: 2019-09-30 09:15:18.0
Author: Jon Evans
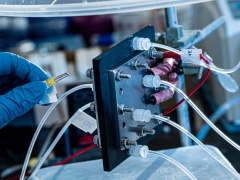
The flow cell with a solid polymer electrolyte
developed by scientists at Rice University.
Photo: Jeff Fitlow/Rice University.
Formic acid (HCOOH) already has a lot of uses, including in tanning and dyeing, as a preservative in animal food, and as an important intermediate in the production of a wide range of industrial chemicals, including toluene and many esters. But it could soon find an even more important role as a liquid storage medium for hydrogen, as it is the simplest molecule that can be formed by combining carbon dioxide (CO2) with molecular hydrogen (H2) (see Sting in the tail).
Such a storage medium is required if vehicles powered by fuel cells running on hydrogen are ever to become a common sight on the roads, because gaseous hydrogen is impractical to store and transport. In contrast, liquid formic acid can be stored and transported using existing tankers and pipelines, and is able to hold nearly 1000 times the energy of the same volume of hydrogen gas. Breaking down the formic acid to release hydrogen would release carbon dioxide as well, but this wouldn’t add to emissions because the released carbon dioxide would simply be taken up by the creation of new formic acid, forming a closed loop.
The trick, obviously, is finding an efficient way to combine carbon dioxide with molecular hydrogen. At the moment, this is generally done in a flow cell, where carbon dioxide and an electrolyte solution flow through a cell separated into several chambers by an ion-exchange membrane and two catalyst-coated gas diffusion electrodes. Powered by electricity, the carbon dioxide is catalytically reduced at the cathode, while the water in the electrolyte solution is split into oxygen and hydrogen at the anode. The reduced carbon dioxide and hydrogen then migrate into a central chamber that also contains the electrolyte, where they react together to produce formic acid.
The problem with this approach is that the formic acid becomes mixed up with the liquid electrolyte, requiring them to be separated from each other, which takes time and energy. So, a team of scientists from the US and Saudi Arabia, led by Haotian Wang at Rice University, decided to try replacing the liquid electrolyte with a solid polymer electrolyte. Several solid electrolytes have already been developed for use in next-generation batteries, giving the scientists a selection to try out.
As they report in a paper in Nature Energy, their flow cell comprises two catalyst-coated gas diffusion electrodes next to two ion-exchange membranes, separated by a solid polymer electrolyte. Carbon dioxide flows past the outside of the cathode, where it is reduced, while a sulfuric acid solution flows past the outside of the anode, where hydrogen ions (protons) are released. The reduced carbon dioxide and hydrogen ions then flow through the ion-exchange membranes to the solid electrolyte, where they combine to form formic acid, Because the electrolyte is porous, this formic acid can simply pass out of the bottom of the cell.
Using this flow cell, Wang and his colleagues found they could continuously generate a pure formic acid solution for 100 hours with an energy conversion efficiency of around 42%. Furthermore, by changing the catalysts on the electrodes, their fuel cell was also able to generate various other useful organic chemicals from carbon dioxide and hydrogen, including acetic acid, ethanol and n-propanol.
Sticking with formic acid, though, a team of Japanese scientists, led by Kohsuke Mori at Osaka University, has found yet another use for it, in the production of deuterium, an isotope of hydrogen that contains a neutron in its atomic nucleus along with the usual proton. Deuterium takes the place of hydrogen in around 0.016% of Earth’s water, known as heavy water, and has a number of uses, especially in the synthesis of various deuterium-labelled compounds. At the moment, deuterium is usually extracted from heavy water by electrolysis, which uses a lot of energy, or by reacting it with metal-based compounds, which generates a lot of waste.
A potentially cleaner and more efficient way would be to replace the deuterium in heavy water with hydrogen, by reacting the heavy water with a hydrogen-rich compound. As Mori and his colleagues report in a paper in Nature Communications, they have now shown this can be done with formic acid. By reacting formic acid with heavy water over a platinum-silver nanocatalyst on a silica substrate modified with amine groups, they were able to release the deuterium.
The views represented here are solely those of the author and do not necessarily represent those of John Wiley and Sons, Ltd. or of the SCI.
Displaying 2 keywords used to tag this article:
- DG6582 Mens Moncler Down Jackets Gr
- Jules Audemars-Australia Best Quali
